Abstract
Introduction: Chronic myelomonocytic leukemia (CMML) and primary myelofibrosis (MF) are distinct myeloid malignancies with clinical and pathologic features that often overlap. The presence of significant bone marrow fibrosis with concomitant monocytosis can be diagnostically challenging. Distinguishing between these two clinical entities has important prognostic and therapeutic implications. In this study, we aimed to genomically characterize cases with overlapping features of both fibrosis and monocytosis and describe their clinical outcomes. Then, using well-established CMML and MF databases, we aimed to identify disease-specific genomic abnormalities to allow for improved diagnostic characterization.
Methods: Including molecularly annotated patients (pts) from our CMML and MF databases, we created 3 cohorts. Cohort 1 was comprised of MF pts without significant monocytosis. Cohort 2 included CMML pts with absent/minimal bone marrow fibrosis. Cohort 3 included CMML pts with fibrosis and MF pts with monocytosis. Significant fibrosis defined as grade 2-3 by European consensus recommendations. Monocytosis defined as an absolute (>800/µL, the upper limit of normal in our laboratory) and relative (>10%) peripheral monocyte count. Cohorts 1 and 2 were compared to establish disease-specific somatic gene mutation patterns. Enriched variables were those that occurred significantly more often with p < 0.05. Specificity threshold of > 95% was used. Blinded pathological review of overlap bone marrow cases revealed concordance with original diagnosis in > 95% of cases.
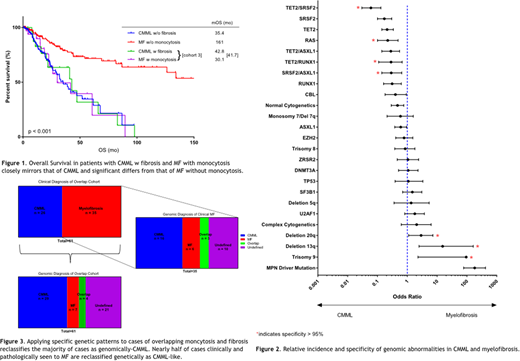
Results: Cohorts 1, 2 and 3 were comprised of 181, 168 and 61 pts, respectively. Among 61 pts in cohort 3, 26 had a prior CMML diagnosis and 35 had prior MF diagnosis. Median OS (mOS) in cohort 1 was 161 months (mo) compared to 35 mo in cohort 2 (p < 0.001) and 42 mo in cohort 3 (p < 0.001). mLFS for cohorts 1, 2, and 3 were not reached, 61 mo and 42 mo, respectively (p < 0.001).
We compared molecular and cytogenetic abnormalities between cohort 1 and 2, assessing individual and commonly co-occurring abnormalities. Genomic abnormalities more common in CMML were mutations in TET2 (p < 0.001), RAS (p < 0.001), RUNX1 (p < 0.001), SRSF2 (p < 0.001), CBL (p = 0.05), ASXL1 (p = 0.04), TET2/SRSF2 (p < 0.001), TET2/ASXL1 (p < 0.001), TET2/RUNX1 (p = 0.03), SRSF2/ASXL1 (p = 0.004), and normal karyotype (p = 0.002). Genomic abnormalities more common in MF included driver mutations (i.e. JAK2, MPL, or CALR) (p < 0.001), del 20q (p = 0.03), del 13q (p < 0.001), and trisomy 9 (p = 0.004). Disease-specific abnormalities were those that were enriched in either CMML or MF with a specificity of >95%. CMML-specific abnormalities included RAS mutations and co-mutations in TET2/RUNX1, TET2/SRSF2, and SRSF2/ASXL1. MF-specific genomic abnormalities included del20q, del13q, and trisomy 9.
We applied these disease-specific genomic abnormalities to cohort 3 to see if these findings could stratify pts toward a CMML-like genotype or MF-like genotype. Of 61 patients, 29 displayed only CMML-like genomic features (genomic CMML), 7 only MF-like features (genomic MF), 4 had both CMML and MF-specific features (genomic overlap) and 21 genomically undefined. Among those in cohort 3 with clinical MF diagnosis, 16 (46%) were reclassified as genomic CMML, 6 (17%) as genomic MF, 3 (9%) as genomic overlap, and 10 (31%) as genomically undefined. Among those with an original clinical diagnosis of CMML, 1 was redefined as genomic MF. OS for genomic CMML did not differ from genomic MF (p = 0.70). There was a trend for inferior LFS in genomic CMML compared to genomic MF (40 mo vs 59 mo, p = 0.19). Multivariate analysis identified the strongest prognostic features in cohort 3 as age > 70 (OR 9.4, p = 0.05), platelet count < 100,000/µL (OR 4.6, p = 0.02) and degree of bone marrow fibrosis (OR 5.1, p = 0.009).
Conclusions: Specific genomic features distinguish CMML from MF. Application of these findings to pts with overlapping clinicopathologic features provides clarity in >50% of cases, primarily reclassifying patients as CMML. Clinically, outcomes in this overlap group with bone marrow fibrosis and monocytosis mirror those of CMML, regardless of genomic assignment; however, the presence of thrombocytopenia and magnitude of bone marrow fibrosis provide further prognostic discrimination. Future studies testing CMML-like therapeutic strategies should be considered in MF with monocytosis.
Kuykendall:Celgene: Honoraria; Janssen: Consultancy. Sweet:BMS: Honoraria; Celgene: Honoraria, Speakers Bureau; Jazz: Speakers Bureau; Astellas: Consultancy; Phizer: Consultancy; Novartis: Consultancy, Honoraria, Speakers Bureau; Agios: Consultancy; Jazz: Speakers Bureau; Agios: Consultancy; Phizer: Consultancy; Astellas: Consultancy; Novartis: Consultancy, Honoraria, Speakers Bureau; BMS: Honoraria; Celgene: Honoraria, Speakers Bureau. Sallman:Celgene: Research Funding, Speakers Bureau. List:Celgene: Research Funding. Komrokji:Novartis: Honoraria, Speakers Bureau; Celgene: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Novartis: Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal